The Nitrogen Cycle – Environment Notes – For W.B.C.S. Examination.
নাইট্রোজেন চক্র – পরিবেশের নোট – WBCS পরীক্ষা।
Nitrogen is one of the primary nutrients critical for the survival of all living organisms. Although nitrogen is very abundant in the atmosphere, it is largely inaccessible in this form to most organisms. This article explores how nitrogen becomes available to organisms and what changes in nitrogen levels as a result of human activity means to local and global ecosystems.Continue Reading The Nitrogen Cycle – Environment Notes – For W.B.C.S. Examination.
Nitrogen is one of the primary nutrients critical for the survival of all living organisms. It is a necessary component of many biomolecules, including proteins, DNA, and chlorophyll. Although nitrogen is very abundant in the atmosphere as dinitrogen gas (N2), it is largely inaccessible in this form to most organisms, making nitrogen a scarce resource and often limiting primary productivity in many ecosystems. Only when nitrogen is converted from dinitrogen gas into ammonia (NH3) does it become available to primary producers, such as plants.
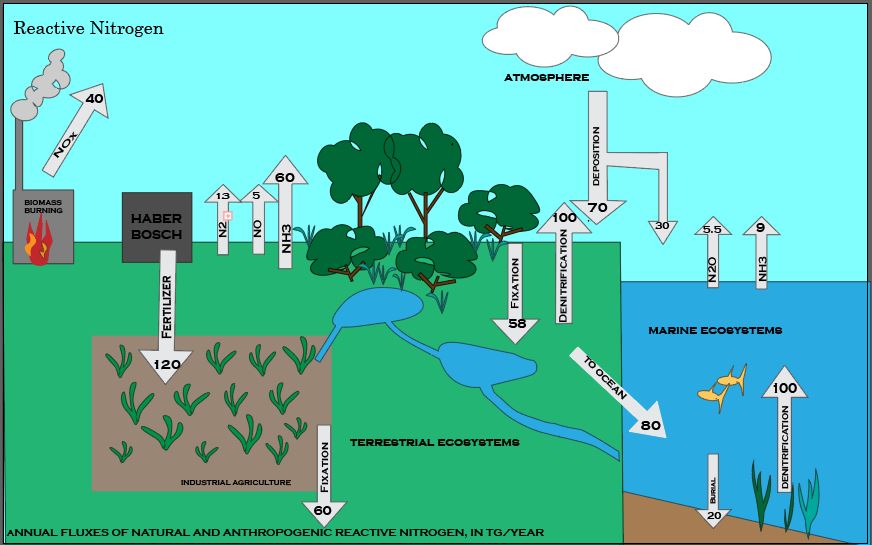
In addition to N2 and NH3, nitrogen exists in many different forms, including both inorganic (e.g., ammonia, nitrate) and organic (e.g., amino and nucleic acids) forms. Thus, nitrogen undergoes many different transformations in the ecosystem, changing from one form to another as organisms use it for growth and, in some cases, energy. The major transformations of nitrogen are nitrogen fixation, nitrification, denitrification, anammox, and ammonification (Figure 1). The transformation of nitrogen into its many oxidation states is key to productivity in the biosphere and is highly dependent on the activities of a diverse assemblage of microorganisms, such as bacteria, archaea, and fungi.
Nitrogen Fixation
Nitrogen gas (N2) makes up nearly 80% of the Earth’s atmosphere, yet nitrogen is often the nutrient that limits primary production in many ecosystems. Why is this so? Because plants and animals are not able to use nitrogen gas in that form. For nitrogen to be available to make proteins, DNA, and other biologically important compounds, it must first be converted into a different chemical form. The process of converting N2 into biologically available nitrogen is called nitrogen fixation. N2 gas is a very stable compound due to the strength of the triple bond between the nitrogen atoms, and it requires a large amount of energy to break this bond. The whole process requires eight electrons and at least sixteen ATP molecules (Figure 2). As a result, only a select group of prokaryotes are able to carry out this energetically demanding process. Although most nitrogen fixation is carried out by prokaryotes, some nitrogen can be fixed abiotically by lightning or certain industrial processes, including the combustion of fossil fuels.
Nitrification
Nitrification is the process that converts ammonia to nitrite and then to nitrate and is another important step in the global nitrogen cycle. Most nitrification occurs aerobically and is carried out exclusively by prokaryotes. There are two distinct steps of nitrification that are carried out by distinct types of microorganisms. The first step is the oxidation of ammonia to nitrite, which is carried out by microbes known as ammonia-oxidizers. Aerobic ammonia oxidizers convert ammonia to nitrite via the intermediate hydroxylamine, a process that requires two different enzymes, ammonia monooxygenase and hydroxylamine oxidoreductase (Figure 4). The process generates a very small amount of energy relative to many other types of metabolism; as a result, nitrosofiers are notoriously very slow growers. Additionally, aerobic ammonia oxidizers are also autotrophs, fixing carbon dioxide to produce organic carbon, much like photosynthetic organisms, but using ammonia as the energy source instead of light.
Anammox
Traditionally, all nitrification was thought to be carried out under aerobic conditions, but recently a new type of ammonia oxidation occurring under anoxic conditions was discovered (Strous et al. 1999). Anammox (anaerobic ammonia oxidation) is carried out by prokaryotes belonging to the Planctomycetes phylum of Bacteria. The first described anammox bacterium was Brocadia anammoxidans. Anammox bacteria oxidize ammonia by using nitrite as the electron acceptor to produce gaseous nitrogen . Anammox bacteria were first discovered in anoxic bioreactors of wasterwater treatment plants but have since been found in a variety of aquatic systems, including low-oxygen zones of the ocean, coastal and estuarine sediments, mangroves, and freshwater lakes. In some areas of the ocean, the anammox process is considered to be responsible for a significant loss of nitrogen (Kuypers et al. 2005). However, Ward et al. (2009) argue that denitrification rather than anammox is responsible for most nitrogen loss in other areas. Whether anammox or denitrification is responsible for most nitrogen loss in the ocean, it is clear that anammox represents an important process in the global nitrogen cycle.
Denitrification
Denitrification is the process that converts nitrate to nitrogen gas, thus removing bioavailable nitrogen and returning it to the atmosphere. Dinitrogen gas (N2) is the ultimate end product of denitrification, but other intermediate gaseous forms of nitrogen exist (Figure 7). Some of these gases, such as nitrous oxide (N2O), are considered greenhouse gasses, reacting with ozone and contributing to air pollution.
Unlike nitrification, denitrification is an anaerobic process, occurring mostly in soils and sediments and anoxic zones in lakes and oceans. Similar to nitrogen fixation, denitrification is carried out by a diverse group of prokaryotes, and there is recent evidence that some eukaryotes are also capable of denitrification (Risgaard-Petersen et al. 2006). Some denitrifying bacteria include species in the genera Bacillus, Paracoccus, and Pseudomonas. Denitrifiers are chemoorganotrophs and thus must also be supplied with some form of organic carbon.
Denitrification is important in that it removes fixed nitrogen (i.e., nitrate) from the ecosystem and returns it to the atmosphere in a biologically inert form (N2). This is particularly important in agriculture where the loss of nitrates in fertilizer is detrimental and costly. However, denitrification in wastewater treatment plays a very beneficial role by removing unwanted nitrates from the wastewater effluent, thereby reducing the chances that the water discharged from the treatment plants will cause undesirable consequences (e.g., algal blooms).
Ammonification
When an organism excretes waste or dies, the nitrogen in its tissues is in the form of organic nitrogen (e.g. amino acids, DNA). Various fungi and prokaryotes then decompose the tissue and release inorganic nitrogen back into the ecosystem as ammonia in the process known as ammonification. The ammonia then becomes available for uptake by plants and other microorganisms for growth.
Our own publications are available at our webstore (click here).
For Guidance of WBCS (Exe.) Etc. Preliminary , Main Exam and Interview, Study Mat, Mock Test, Guided by WBCS Gr A Officers , Online and Classroom, Call 9674493673, or mail us at – mailus@wbcsmadeeasy.in
Visit our you tube channel WBCSMadeEasy™ You tube Channel
Please subscribe here to get all future updates on this post/page/category/website



 +919674493673
+919674493673  mailus@wbcsmadeeasy.in
mailus@wbcsmadeeasy.in